Cell therapy operates within one of the most complex IP environments. For innovators and investors, navigating this landscape is critical. The ability to commercialise a product without infringing on the rights of others is not a single hurdle, but a multi-layered challenge requiring strategic freedom to operate (FTO) analysis. Concurrently, innovators face the distinct but related challenge of building a strong and robust patent portfolio that protects their own technology, protects the market and secures return of investment.
Importantly, from the point of view of securing investment and partnering, FTO and patentability analysis cannot be late-stage legal checks. Both FTO and invention spotting must be integrated as core components of business strategy from the earliest stages of R&D, clinical development all the way up to launch and beyond. In this rapidly advancing field, a proactive IP strategy that anticipates market dynamics will be crucial for attracting investment and ensuring these transformative therapies reach patients. The most successful companies will be those that use proactive diligence to understand the competitive landscape, innovate to solve unaddressed needs in the industry, and build their own robust IP portfolios.
A question of permission, not possession: Patentability versus FTO
When talking with scientists, one of the most frequently asked questions and areas of confusion is the difference between freedom to operate (FTO) and patentability. The distinction is highly relevant to the field of biotechnology and understanding it is crucial (Evolve Insights).
The question of patentability asks whether an invention is new, inventive and supported by the data. Importantly, if a patent is granted for an invention, it gives the patent owner the right to exclude others from making, using, or selling the patented invention. However, owning a patent does not automatically grant the patentee the right to practise their own invention. This is where freedom to operate comes in. FTO addresses a different and equally critical question, namely can you commercialise a product without infringing on the valid patent rights of others. A situation may arise where an invention is patentable but nonetheless falls within the scope of protection of an existing foundational patent. In this case, the invention may be patentable (new and non-obvious) but the inventors of the new patent may lack freedom to operate for their invention in view of the existing patent.
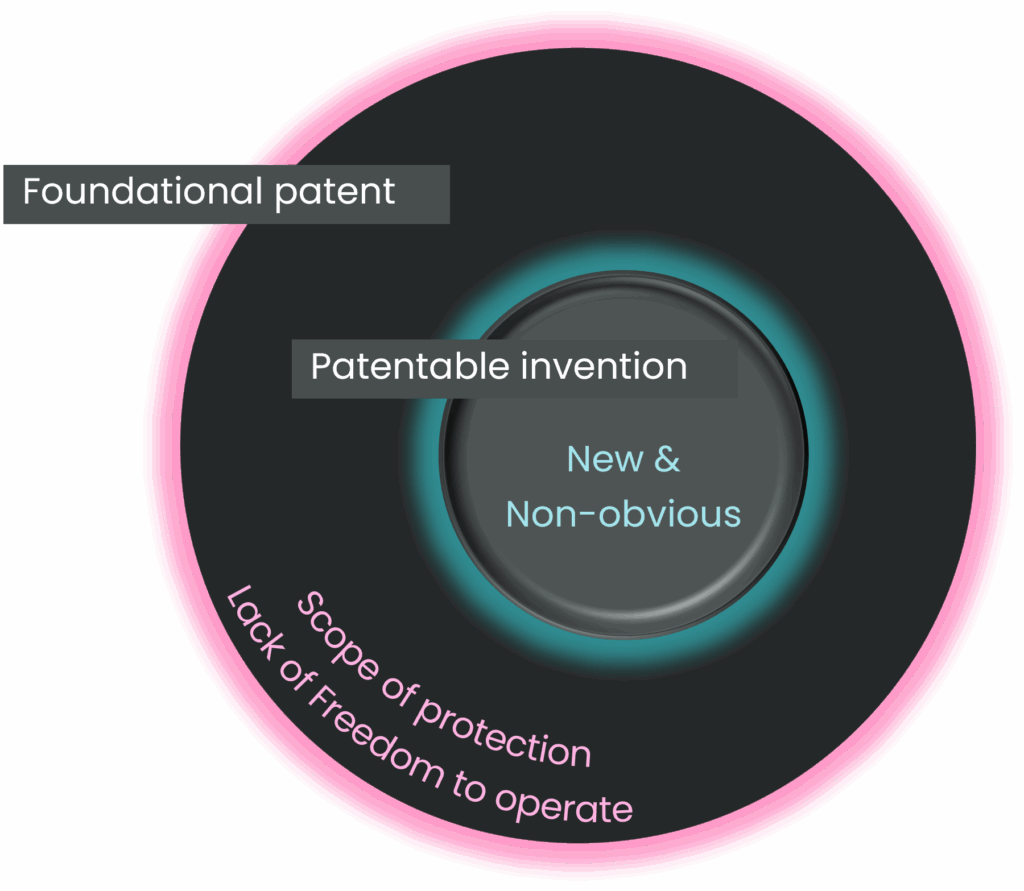
The distinction between FTO and patentability is particularly important in cell therapy. A cell therapy biotech company might, for example, develop and patent a novel Chimeric Antigen Receptor (CAR) with an inventive new co-stimulatory domain that shows unexpected T-cell persistence. The invention of the new co-stim domain is likely patentable. However, if that CAR borrows a target-binding domain or scFv sequence that is already covered by a third-party CAR or even antibody patent, the company may lack freedom to operate. Indeed, antibody patents often define antibodies only by the binding domain and are not limited to specific constant regions. To bring its product to market, the company may need a licence from the scFv patent holder.
FTO issues are often particularly acute for cell therapy pipeline products using multiple platform technologies. A company focused on developing a select number of therapeutic products may need to incorporate a number of different platforms, including gene editing tools or unique vector systems. A pipeline product may need to navigate the FTO landscape for each of the components used in the product, potentially requiring multiple licences that can lead to a commercially burdensome stacking of royalty obligations.
Navigating a complex, competitive and crowded landscape
The cell therapy sector arguably presents an FTO landscape of unparalleled complexity in biotech. A commercially viable product requires a clear path through the distinct and often overlapping patent landscapes of its fundamental components. Deconstructing the product into its constituent parts allows for a focused analysis of the specific IP risks.
A single CAR-T cell product, for example, embodies a cellular vehicle (the T cell), a gene editing platform, a delivery vector, a therapeutic construct, and a sophisticated manufacturing process. Each of these modules may present its own dense patent landscape (Evolve Insights). Foundational patents, often originating from academic institutions, cover core technologies, whilst a plethora of subsequent patents from commercial entities creates a complex competitive landscape that can be difficult to navigate.
The specific FTO and patentability challenges are also profoundly shaped by the therapeutic modality being pursued. The race for allogeneic “off-the-shelf” therapies, which use cells from a single healthy donor, is dominated by foundational platform patents on the technologies that make this scalable model possible. Key FTO battlegrounds include the cell source, particularly induced pluripotent stem cells (iPSCs), and the immune evasion engineering strategies required to prevent immune rejection. Many of the genetic modifications for hypo-immunogenicity, for example, are very well known in the field (and therefore non-patentable) and/or protected by broad foundational IP. New patentable approaches that avoid the existing IP should focus on identifying new edits that are, for example, cell-type specific (e.g. downregulatory of cell-specific markers), more cost-efficient and scalable than previous approaches, or provide more effective and robust evasion of host immunity.
The CAR-T field in general (auto and allo) also represents a particularly interesting FTO and patentability challenge. Many companies in the space are pursuing the same targets (BCMA, CD19, CD20 etc.), albeit with different platform technologies. Demonstrating the patentability of a new BCMA CAR can therefore be a challenge, particularly in Europe, where to achieve patent protection, it may be necessary to show what problem the new CAR solves in view of the existing BCMA CARs that are already known. Additionally, some of the established players in the field may have filed early field-blocking IP for known targets in particular indications.
Looking further ahead, the potentially revolutionary modality of in vivo cell therapy, where genetic engineering occurs inside the body, presents a new set of FTO and patentability challenges. From the patentability perspective, many in vivo strategies may reuse existing CARs or delivery vectors. New IP will have to be found in order to provide exclusivity for these next generation products after the CAR product IP expires. From the FTO perspective, many of the delivery vehicles for in vivo CAR-T make use of existing technologies with complex IP landscapes. LNPs are a particular example of a complex FTO landscape, with the LNP patent wars already ongoing in the vaccine sector. There are nonetheless also considerable IP opportunities in the in vivo cell therapy space, given the need for (and considerable challenge of) specific cell targeting (Evolve Insights)..
FTO strategies for cell therapy
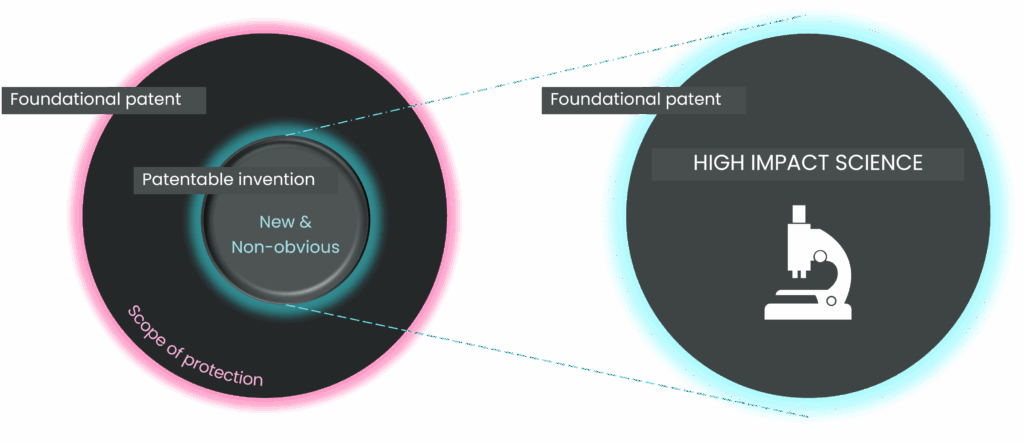
So, how can innovators in the cell therapy sector best navigate this combined minefield of freedom to operate and patentability? Faced with a complex patent landscape, there may be a temptation to simply try and design around the existing IP with minor modifications. However, whilst this strategy may help with FTO, it is unlikely to lead to strong patentability. Counterintuitively, the best strategy is, in fact, not to focus solely on the IP. Instead, innovative science is the best strategy for achieving both patentability and FTO. By focusing on solving a genuine problem in the industry, and not just making incremental changes to existing technology, innovators are more likely to create truly inventive solutions, based on high impact science, that are far removed from existing IP. The focus for any business plan should be scientific and commercial problem solving, and not navigating legal constraints.
It is also important to remember that not every granted patent is enforceable. Some broad, field-blocking patents may be old and expire before a new product gets to market, or they may be vulnerable to an invalidity challenge. The legal requirements for patentability can be stringent. For instance, the US “written description” requirement demands that a patent specification demonstrate that the inventors were in “possession” of the full scope of what they claimed at the time of filing. Recent court decisions have established a much higher bar for satisfying this requirement for biologics, including chimeric antigen receptors (Evolve Insights). In the case of Juno v Kite, for example, the US Court of Appeals for the Federal Circuit (CAFC) invalidated BMS/Juno’s broad CAR-T patent for a CAR co-stimulatory domain. The patent was not limited to any specific target or scFv binding sequence. The court found that the patent was not supported by sufficient written description of the invention because of the large number of potential CAR targets covered. Since this decision, the US Supreme Court has also confirmed the very high bar for the patentability of broad claims directed to biologics (Evolve Insights).
Many granted broad patents in the cell therapy field may therefore not be defensible today because their disclosure would be seen as a mere research plan rather than a description of a fully realised invention. This potentially creates opportunities for innovators who can show that significant inventive activity was needed to arrive at their own solution.
Final thoughts
To secure investment in cell therapies, it is essential to have a clear IP strategy that ensures a sufficient exclusivity period to provide a return on investment. The unique nature of these products creates both challenges and opportunities. Unlike traditional pharmaceuticals, where loss of exclusivity is more clearly defined, cell therapies require a more creative and forward-thinking IP strategy.
At Evolve, we are experts in IP strategy for cell therapies. We have extensive experience developing and coordinating IP strategy for cell therapy with regulatory and manufacturing business functions. Do you need advice on how to protect your cell therapy innovation? We would love to hear from you. Learn more about IP strategy for cell and gene therapies by registering for our upcoming webinar, or subscribe to our newsletter for the latest insights.
Author: Rose Hughes
Further reading
- Securing market protection for cell therapies: Patents versus regulatory exclusivity
- Beyond the process: Securing robust IP protection for cell therapies
- Strict US written description requirement applied to CAR-T-cell therapy (Juno v Kite)
- Pitfalls of cell therapy manufacturing IP – A case study (T 0868/23)
- IP strategy for the universal cell therapy revolution
- Freedom to operate versus patentability in biotech: What the difference is and why it matters
- US Supreme Court decision in Amgen v Sanofi: The European Perspective